Bonsai Nut
Nuttier than your average Nut
The term ALKALINE in the context of this discussion means having a pH greater than 7.
The term ALKALINITY is the capacity of water to buffer changes in pH.
The two terms do NOT refer to the same thing and cannot be used interchangeably. My pool water can be alkaline, and have high or low alkalinity. To increase alkalinity, I will add sodium bicarbonate. To make the water less alkaline, I will add hydrochloric acid. If the water has high alkalinity, I have to add more acid to change the pH than if the water has low alkalinity.
Very confusing.
In the case of plants, you usually want to avoid water that is both ALKALINE and has high ALKALINITY. Water that is alkaline with low alkalinity doesn't have much ability to neutralize acidity in the soil. Water that is alkaline and has HIGH alkalinity has a greater ability to neutralize acid in the soil and increase soil pH. The more alkaline the water and the higher the water alkalinity, the greater this impact will be.
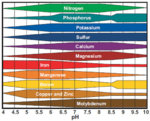
At pH above 7.0 (neutral) plants will have greater difficulty taking up specific trace elements. This impact can be much more noticeable when growing plants in containers and/or with some types of inorganic soils. One of the first signs (at least here on the west coast) is chlorosis caused by iron deficiency - even though the soil when tested has plenty of iron in it.
A good read:
Water Quality: pH and Alkalinity
The term ALKALINITY is the capacity of water to buffer changes in pH.
The two terms do NOT refer to the same thing and cannot be used interchangeably. My pool water can be alkaline, and have high or low alkalinity. To increase alkalinity, I will add sodium bicarbonate. To make the water less alkaline, I will add hydrochloric acid. If the water has high alkalinity, I have to add more acid to change the pH than if the water has low alkalinity.
Very confusing.
In the case of plants, you usually want to avoid water that is both ALKALINE and has high ALKALINITY. Water that is alkaline with low alkalinity doesn't have much ability to neutralize acidity in the soil. Water that is alkaline and has HIGH alkalinity has a greater ability to neutralize acid in the soil and increase soil pH. The more alkaline the water and the higher the water alkalinity, the greater this impact will be.
At pH above 7.0 (neutral) plants will have greater difficulty taking up specific trace elements. This impact can be much more noticeable when growing plants in containers and/or with some types of inorganic soils. One of the first signs (at least here on the west coast) is chlorosis caused by iron deficiency - even though the soil when tested has plenty of iron in it.

A good read:
Water Quality: pH and Alkalinity
Last edited:

